BACKGROUND: Central nervous system (CNS) relapse in systemic non-Hodgkin's lymphoma is associated with a guarded prognosis with a median survival of only 2-6 months. However, information regarding CNS relapse is still limited and there is no consensus regarding which clinical or biologic risk factors should be used to identify patients who need prophylaxis and the optimal prophylactic regimen has not yet been established. The CNS-IPI score represents a robust risk model for CNS relapse in patients with diffuse large B cell lymphoma treated with R-CHOP and may potentially serve as a useful benchmark to evaluate the impact of novel treatment approaches. In particular, patients with chromosomal rearrangements of MYC and BCL2 and/or BCL6 genes, as is encountered in double hit (DHL) or triple hit (THL) lymphoma, have been associated with an increased risk for CNS involvement in retrospective series. CNS prophylaxis is typically administered in patients with DHL/THL treated with DA-R-EPOCH due to this perceived heightened risk, but there are limited data to support this approach.
METHODS: We conducted a comprehensive multi-institutional retrospective analysis of outcomes of patients with DHL/THL, who received a minimum of 4 cycles of DA-R-EPOCH therapy with or without CNS prophylaxis, namely intrathecal (IT) or high-dose intravenous (IV) methotrexate or cytarabine. Each academic institution provided clinical data to assess the risk of CNS relapse (CNS-IPI) and FISH testing for MYC/BCL2/BCL6 translocations. Outcomes included overall survival (OS), progression-free survival (PFS), CNS relapse-free survival (CNSFS) and time to CNS recurrence which were calculated from time of diagnosis. Fisher's exact test or Chi-square test and Kaplan-Meier method were used to evaluate associations between outcomes and demographics as well as clinical characteristics including administration of CNS prophylaxis and CNS-IPI risk stratification.
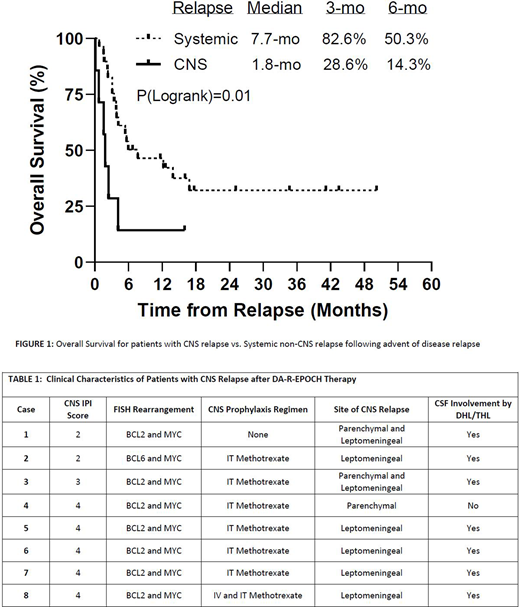
RESULTS: We collected data on 109 DHL/THL patients across 6 US academic medical centers. Demographic and clinical variables at diagnosis include - Age >60 57.8%(n=63); Male sex 59.6%(n=65); Stage III/IV 78.9%(n=86); ECOG PS >1 12.8%(n=14); CNS-IPI low risk (score 0-1) 11%(n=12), intermediate risk (score 2-3) 57.8%(n=63), high risk (score 4-5) 31.2%(n=34). A total of 95 (87.2%) patients received CNS prophylaxis [IT 81.1%(n=77); IV ± IT 18.9%(n=18)] with a median of 4 doses. Eight patients developed CNS relapse (7.3%) during a median of 25.9 (range 4.0 to 63.2) months of follow up. The patterns of relapse were predominantly leptomeningeal (n=7/8), as shown in Table 1. Estimated incidence was 4.9% at 12 months and 6.1% at 24 months after diagnosis. 5.6% (1/18) of the patients who received IV ±IT CNS prophylaxis developed CNS relapse, whereas the rates of CNS recurrence for patients who received only IT or no CNS prophylaxis were 6.5% and 7.1% respectively. No statistically significant differences in outcomes were found between baseline patient characteristics with respect to whether or not CNS prophylaxis was received, as well as the route of administration of CNS prophylaxis. The rate of CNS relapse was low for patients with low to intermediate compared to high CNS-IPI scores (4.0% vs. 14.7%, p=0.047). Patients with high CNS-IPI scores had reduced CNSFS (2-yr survival: 85.2% vs 97.3%, p=0.08), as well as reduced OS (2-yr survival: 46.5% vs. 87.5%, p<0.001) and PFS (47.1% vs 82.6%, p=0.001). As shown in Figure 1, when comparing OS from the advent of disease relapse, OS after CNS relapse was significantly worse than OS after systemic non-CNS relapse.
CONCLUSIONS: CNS relapse is associated with significantly reduced OS as compared to systemic non-CNS relapse following R-EPOCH treatment in DHL/THL, justifying the need for a rational CNS prophylaxis strategy in these patients. The rate of CNS relapse was significantly lower in patients in the low-intermediate CNS-IPI risk category but it is unclear whether CNS events are reduced by administration of IT CNS prophylaxis due to the relatively small proportion of DHL/THL patients who did not receive any CNS prophylaxis. Furthermore, only 1 of the 18 DHL/THL patients (including 6 patients with high CNS-IPI scores) who received IV ± IT CNS prophylaxis developed CNS relapse, suggesting that this prophylactic strategy, especially in patients with high risk CNS-IPI scores, may be more effective in preventing this devastating clinical event.
Haverkos:Viracta THerapeutics: Consultancy. Hughes:Acerta Pharma and HOPA: Research Funding; AstraZeneca: Consultancy; Genzyme: Consultancy; Janssen: Consultancy; AbbVie: Consultancy. Grover:Genentech: Research Funding; Tessa: Consultancy. Portell:AbbVie: Research Funding; Amgen: Consultancy; Bayer: Consultancy; TG Therapeutics: Research Funding; Infinity: Research Funding; Roche/Genentech: Consultancy, Research Funding; Xencor: Research Funding; BeiGene: Consultancy, Research Funding; Kite: Consultancy, Research Funding; Acerta/AstraZeneca: Research Funding; Janssen: Consultancy; Pharmacyclics: Consultancy. Voorhees:AstraZeneca: Research Funding. Landsburg:Triphase: Research Funding; Seattle Genetics: Speakers Bureau; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Kamdar:BMS: Consultancy; Seattle Genetics: Speakers Bureau; Pharmacyclics: Consultancy; AstraZeneca: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; Abbvie: Consultancy. Kahl:Acerta: Consultancy, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche Laboratories Inc: Consultancy; Pharmacyclics LLC: Consultancy; Genentech: Consultancy; Celgene Corporation: Consultancy; AstraZeneca Pharmaceuticals LP: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Hill:Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; AstraZenica: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal